Abstract and Introduction
Abstract
Graphical Abstract
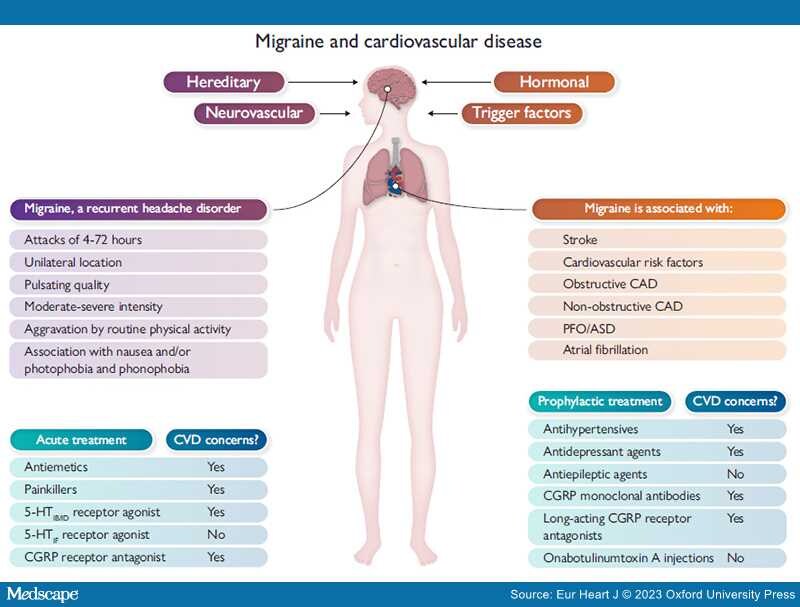
Migraine and cardiovascular disease. Factors influencing the occurrence of migraine, the International Classification of Headache Disorders, 3rd edition, definition of migraine (in purple), cardiovascular diseases associated with migraine (in orange) and treatment options for migraine (in blue). Abbreviations: 5-HT, 5-hydroxytryptamine; ASD, atrial septal defect; CAD, coronary artery disease; CGRP, calcitonin gene-related peptide; CVD, cardiovascular disease; PFO, patent foramen ovale. Prevalence of migraine according to sex and age from Lancet Neurol 2008;7:354–61. https://doi.org/10.1016/S1474-4422(08)70062-0.
Migraine is a chronic neurovascular disease with a complex, not fully understood pathophysiology with multiple causes. People with migraine suffer from recurrent moderate to severe headache attacks varying from 4 to 72 h. The prevalence of migraine is two to three times higher in women compared with men. Importantly, it is the most disabling disease in women <50 years of age due to a high number of years lived with disability, resulting in a very high global socioeconomic burden. Robust evidence exists on the association between migraine with aura and increased incidence of cardiovascular disease (CVD), in particular ischaemic stroke. People with migraine with aura have an increased risk of atrial fibrillation, myocardial infarction, and cardiovascular death compared with those without migraine. Ongoing studies investigate the relation between migraine and angina with non-obstructive coronary arteries and migraine patients with patent foramen ovale. Medication for the treatment of migraine can be preventative medication, such as beta-blockers, angiotensin-converting enzyme inhibitors/angiotensin receptor blockers, antiepileptics, antidepressants, some of the long-acting calcitonin gene-related peptide receptor antagonists, or monoclonal antibodies against calcitonin gene-related peptide or its receptor, or acute medication, such as triptans and calcitonin gene-related peptide receptor antagonists. However, these medications might raise concerns when migraine patients also have CVD due to possible (coronary) side effects. Specifically, knowledge gaps remain for the contraindication to newer treatments for migraine. All cardiologists will encounter patients with CVD and migraine. This state-of-the-art review will outline the basic pathophysiology of migraine and the associations between migraine and CVD, discuss current therapies, and propose future directions for research.
Introduction
The World Health Organization (WHO) stated in 2016 that headache has been underestimated, underrecognized, and undertreated throughout the world.[1] Migraine affects at least 1 billion people worldwide and results in a large global socioeconomic burden. According to the Global Burden of Disease Study 2019 (GBD2019), migraine persists as the second most disabling cause worldwide (after low back pain) and remains number one in women <50 years of age.[2,3] The GBD2019 showed once more that migraine is more prevalent in women compared with men.[4]
The 2021 European Society of Cardiology (ESC) guidelines on cardiovascular disease (CVD) prevention recommend that migraine with aura should be considered in CVD risk assessment.[5] The QRISK3 score, a tool to assess the 10-year risk of CVD, has incorporated migraine in the score.[6] There is a growing interest in the link between migraine and CVD. Basic background information on the pathophysiology of migraine is presented in this paper. Associations between migraine and different types of CVD have been established, such as stroke, obstructive coronary artery disease (CAD), including myocardial infarction (MI), atrial fibrillation (AF), and cardiovascular death. Knowledge on migraine and ischaemia with non-obstructive coronary arteries (INOCA), patent foramen ovale (PFO), or atrial septal defects (ASD) is still the focus of new research.[7] Acute and preventative medications for patients with migraine and CVD are discussed, and data on possible contraindications for their use are presented in this review.
This state-of-the-art review aims to provide background information on the disorder migraine and its associations with CVD based on current data, summarize treatment dilemmas in patients with migraine and CVD, and present knowledge gaps on this topic.
Eur Heart J. 2023;44(30):2815-2828. © 2023 Oxford University Press
Copyright 2007 European Society of Cardiology. Published by Oxford University Press. All rights reserved.