Abstract and Introduction
Abstract
Background: From consumption of fermented foods and probiotics to emerging applications of faecal microbiota transplantation, the health benefit of manipulating the human microbiota has been exploited for millennia. Despite this history, recent technological advances are unlocking the capacity for targeted microbial manipulation as a novel therapeutic.
Aim: This review summarises the current developments in microbiome-based medicines and provides insight into the next steps required for therapeutic development.
Methods: Here we review current and emerging approaches and assess the capabilities and weaknesses of these technologies to provide safe and effective clinical interventions. Key literature was identified through Pubmed searches with the following key words, 'microbiome', 'microbiome biomarkers', 'probiotics', 'prebiotics', 'synbiotics', 'faecal microbiota transplant', 'live biotherapeutics', 'microbiome mimetics' and 'postbiotics'.
Results: Improved understanding of the human microbiome and recent technological advances provide an opportunity to develop a new generation of therapies. These therapies will range from dietary interventions, prebiotic supplementations, single probiotic bacterial strains, human donor-derived faecal microbiota transplants, rationally selected combinations of bacterial strains as live biotherapeutics, and the beneficial products or effects produced by bacterial strains, termed microbiome mimetics.
Conclusions: Although methods to identify and refine these therapeutics are continually advancing, the rapid emergence of these new approaches necessitates accepted technological and ethical frameworks for measurement, testing, laboratory practices and clinical translation.
Introduction
The gastrointestinal (GI) microbiome is known to play an integral role in overall homeostasis; however, alterations can lead to the development and progression of disease. These complex communities contain between 100 and 1000 bacterial species all of which have the ability to interact with the host in different ways. The concept of altering the GI microbiome to improve health outcomes is now well established in modern medicine. Microbiome-based medicines can fall into two categories, microbiome-based biomarkers, and therapeutics (Figure 1). Although some dietary interventions, prebiotics, probiotics, antibiotics and faecal microbiota transplant (FMT) are well-established therapeutics, recent work has raised the possibility of live biotherapeutics, and phage therapies for managing and treating a large array of diseases[1–6] (Figure 1). With the expansion of diverse microbially targeted therapies, coupled with an increasing availability of cost-effective gut metagenomic profiling, it is timely to critically evaluate current capabilities and determine fundamental areas on which to focus future research.
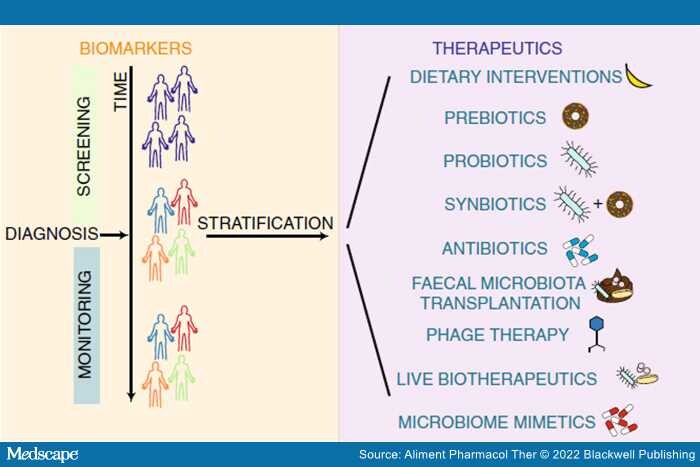
Figure 1.
Overview of the different uses of the microbiome for medicine. Microbiota uses include biomarkers (orange box), where patients are screened monitored and stratified, and therapeutics (purple box), where there are currently nine forms of therapeutics: Dietary interventions, prebiotics, probiotics, synbiotics, antibiotics, faecal microbiota transplantation, phage therapy, live biotherapeutics and microbiome mimetics.
Aliment Pharmacol Ther. 2022;56(2):192-208. © 2022 Blackwell Publishing