Atopic dermatitis (AD) is typically a big issue during early childhood. But it also afflicts up to 5% of adults. Mild cases, which are virtually always managed in primary care, are usually successfully treated with a combination of topical emollients, corticosteroids, and calcineurin inhibitors (tacrolimus, pimecrolimus). In 2016, a new agent entered the armamentarium when the US Food and Drug Administration (FDA) approved a novel topical phosphodiesterase-4 inhibitor (crisaborole) for use in patients ≥ 2 years of age.
In contrast to the anticipated success of this approach for relatively mild disease, these topical agents are not as effective for moderate to severe AD. They are cumbersome to apply to large body surface areas and seldom result in prolonged disease remission.
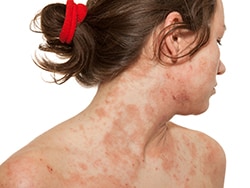
Along comes dupilumab, the first biologic agent to receive FDA approval for the treatment of moderate to severe AD in patients > 12 years of age. The drug is a monoclonal antibody that blocks the proinflammatory signaling pathway that is overactive in atopic disease.[1] Approval followed three pivotal clinical trials, which all demonstrated consistent improvements measured by a range of objective tools.[2,3] Adverse effects were generally mild and included injection-site reactions, headache, nasopharyngitis, and conjunctivitis.[2,3,4] Interestingly, dupilumab had been reported to both improve and induce alopecia areata.[5]
Conjunctivitis, occurring in about 8% of patients, is the most common and treatment-limiting side effect of dupilumab. In trials, risk correlated with AD severity and prior history of conjunctivitis.[6] But will that be the case with more widespread use of the drug in real-world conditions?
A retrospective cohort study of 241 French patients with moderate to severe sought to answer that question.[7]
Results
This most recent trial collected information from clinicians about adult patients treated with the drug over the course of a single year in one of 29 participating centers. Clinicians completed the same tools at baseline and follow-up used to assess efficacy in the trials that led to FDA approval.
The majority (79%) of the patients also used topical corticosteroids during dupilumab therapy.
After a median follow-up of 3.8 months, efficacy outcomes were similar to those seen during the phase 3 clinical trials. However, the incidence of conjunctivitis was much higher (38.2% vs 8%). Conjunctivitis was managed with artificial tears and/or cyclosporine eye drops, depending on severity. Noninfectious ophthalmologic adverse effects resulted in discontinuation of dupilumab in 10 patients.
The other notable adverse event in this real-world trial was hypereosinophilia. Whereas the previous clinical trials showed transient eosinophilia in < 2% of patients, the incidence was fivefold higher in this trial (9.5%). This adverse event was associated with a history of eosinophilia, asthma, and allergic rhinitis—all of which are conditions that would be often seen in patients with AD. Although no patients showed signs of organ involvement, investigators stopped dupilumab therapy in five patients because of persistent hypereosinophilia.
Viewpoint
This real-world cohort study of patients with moderate to severe AD taking dupilumab saw a more than fourfold increase in conjunctivitis compared with previously reported phase 3 trial results. As the investigators acknowledge, the lack of a control group means we should take these results with a big grain of salt (preferably not in the eyes).
The safety profile of dupilumab remains exponentially safer than that of other systemic immunosuppressive alternatives, such as cyclosporine, azathioprine, mycophenolate mofetil, and methotrexate, all of which have been used off-label to manage severe AD.
Nevertheless, we may have underestimated the prevalence of ophthalmologic side effects. It will be interesting to see whether seemingly mild issues, such as noninfectious conjunctivitis, lead to any long-term, more significant ophthalmologic sequelae.
For now, it seems prudent to monitor all patients on dupilumab therapy for signs and symptoms of conjunctivitis and seek ophthalmologic help to manage and monitor even mild ocular inflammation.
Prescribers should also consider checking for hypereosinophilia, although the significance of this reported laboratory finding remains unclear.
Follow Medscape on Facebook, Twitter, Instagram, and YouTube
Medscape Dermatology © 2020 WebMD, LLC
Any views expressed above are the author's own and do not necessarily reflect the views of WebMD or Medscape.
Cite this: Graeme M. Lipper. Dupilumab in the Real World: Beware Adverse Effects - Medscape - Jan 15, 2020.










Comments